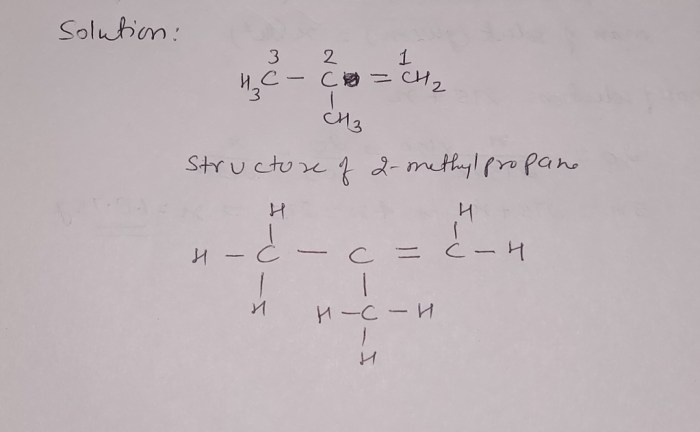Predict the major organic product of the reaction of 2-methyl-1-propene – Predicting the major organic product of the reaction of 2-methyl-1-propene is a fundamental concept in organic chemistry, with applications in various fields. Understanding the factors influencing regio- and stereoselectivity is crucial for accurate prediction and successful synthesis of target compounds.
This comprehensive guide delves into the intricacies of the reaction, exploring Markovnikov’s rule, carbocation stability, steric and electronic effects, and the impact of stereochemistry on the reaction outcome. By examining similar reactions and their outcomes, we gain insights into the factors that govern the formation of specific products.
Reaction of 2-Methyl-1-Propene: Predicting the Major Organic Product
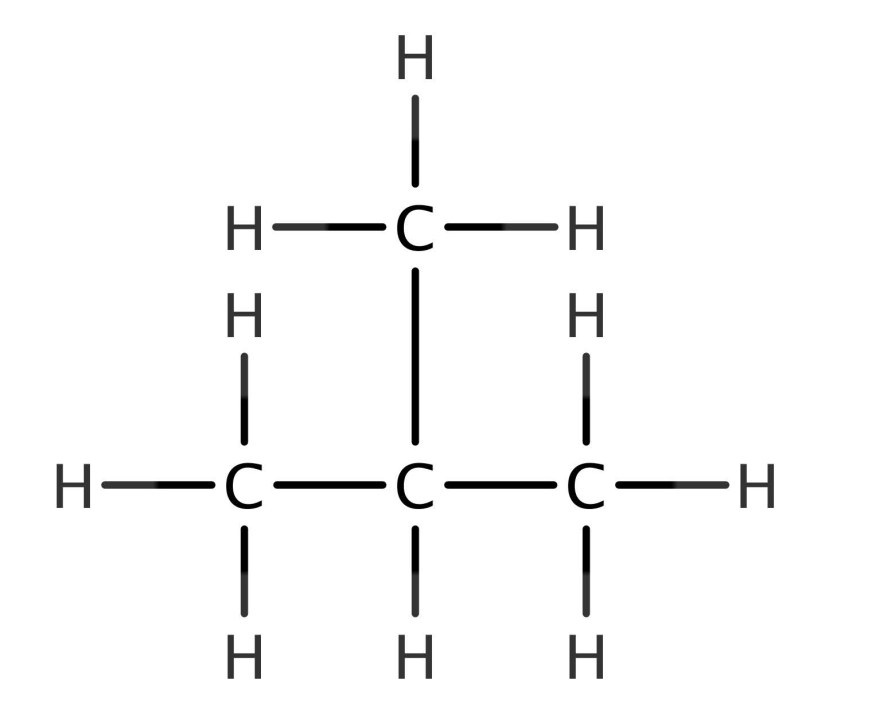
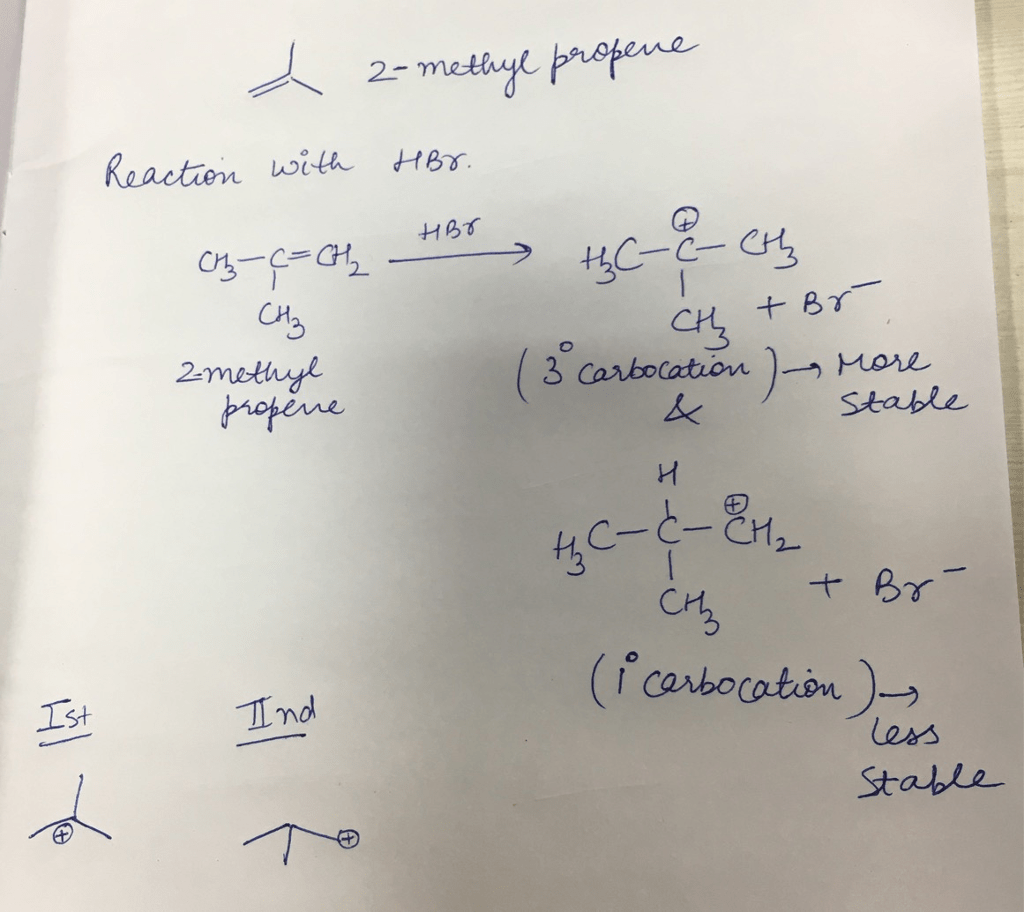
The reaction of 2-methyl-1-propene with a strong electrophile, such as HBr or HCl, leads to the formation of a carbocation intermediate. The stability of this carbocation intermediate determines the regioselectivity of the reaction, which in turn affects the stereochemistry of the product.
Identifying the Major Organic Product
According to Markovnikov’s rule, the major organic product of the reaction is the one that results from the formation of the more stable carbocation intermediate. In the case of 2-methyl-1-propene, the more stable carbocation intermediate is the tertiary carbocation formed by the addition of the electrophile to the carbon bearing the two methyl groups.
The following mechanism illustrates the formation of the major organic product:
- Electrophilic addition:The electrophile (HBr or HCl) adds to the double bond of 2-methyl-1-propene, forming a carbocation intermediate.
- Carbocation rearrangement:The tertiary carbocation intermediate undergoes a rearrangement to form a more stable secondary carbocation.
- Nucleophilic attack:The bromide or chloride ion acts as a nucleophile and attacks the secondary carbocation, forming the major organic product, 2-bromo-2-methylpropane or 2-chloro-2-methylpropane.
Regioselectivity of the Reaction
The regioselectivity of the reaction is influenced by several factors, including:
- Steric effects:Bulky groups, such as methyl groups, hinder the approach of the electrophile to the double bond. This can lead to the formation of the less substituted carbocation intermediate, which is more stable and results in the major organic product.
- Electronic effects:Electron-withdrawing groups, such as halogens, can stabilize carbocations by withdrawing electrons from the positively charged carbon. This can lead to the formation of the more substituted carbocation intermediate, which is more stable and results in the major organic product.
Stereochemistry of the Reaction
The stereochemistry of the product is determined by the stereochemistry of the starting material and the mechanism of the reaction. In the case of 2-methyl-1-propene, the reaction proceeds via a non-stereospecific mechanism, which means that both enantiomers of the product are formed in equal amounts.
Applications of the Reaction, Predict the major organic product of the reaction of 2-methyl-1-propene
The reaction of 2-methyl-1-propene with a strong electrophile is a versatile synthetic tool that can be used to prepare a variety of organic compounds. Some examples include:
- Alkyl halides:The reaction can be used to prepare alkyl halides, which are useful intermediates in organic synthesis.
- Alcohols:The reaction can be used to prepare alcohols by reacting the alkyl halide with a nucleophile, such as hydroxide ion.
- Ethers:The reaction can be used to prepare ethers by reacting the alkyl halide with an alcohol.
Query Resolution: Predict The Major Organic Product Of The Reaction Of 2-methyl-1-propene
What is Markovnikov’s rule?
Markovnikov’s rule states that in the addition of an unsymmetrical alkene to an electrophile, the electrophile adds to the carbon atom of the double bond that has the most hydrogen atoms.
What factors influence the regioselectivity of the reaction?
The regioselectivity of the reaction is influenced by steric effects and electronic effects. Steric effects refer to the hindrance caused by bulky groups, while electronic effects refer to the electron-withdrawing or electron-donating properties of substituents.
How does the stereochemistry of the starting material affect the stereochemistry of the product?
The stereochemistry of the starting material can affect the stereochemistry of the product through stereoelectronic effects. These effects arise from the interaction between the orbitals of the reactants and can lead to the formation of specific stereoisomers.